
RNase as a component of inducible gene expression
Control over gene expression using specific chemical inducers is a favourite method for improving production from bacteria. Most inducible gene expression systems are controlled at the level of transcription. Transcription is one step in gene expression in which information encoded in the DNA is converted to RNA. A later step in gene expression is translation, in which the information in the RNA is converted to protein, the most common ‘workhorse’ of the cell. Unlike transcription, translation has rarely been controllable via inducers. One of my projects is focussed on the use of RNases, which are specialized proteins that rapidly cut and degrade RNA. In this project, a novel RNase has been found whose activity can be controlled by a specific chemical. The unusual ability of this RNase to respond to the chemical will be harnessed as a method for controlling gene expression. If successful, this RNase will be the first of its kind. Furthermore, if translational control can be coupled to transcriptional control, it will open doors to a level of control that has not yet been achievable in science.
Model of an RNase complex – © Vasundra Srinivasan

Increasing PHB accumulation in Cupriavidus necator by increasing cell volume
Cupriavidus necator is THE bacterium most used for industrial production of PHB, a fully biodegradable polymer that can replace some forms of plastic in every-day-life. Under the right conditions, this bacterium stores PHB as an energy reserve. The bacterium is so good at storing PHB that up to 90% of its cell volume is filled with PHB granules. This is remarkable given that the cell also needs space for its DNA, RNA, and proteins, including ribosomes. So how can C. necator possibly produce even more PHB?
One of my projects looks as using controlled gene expression to force the cells to increase their length by up to 50 times (up to 100 µm). Would this increase their PHB production?

Production of curdlan from waste
Curdlan is a valuable product of Agrobacterium tumefaciens, a bacterium that normally lives in plant tissue, occasionally as a plant pathogen. Mostly, curdlan is produced in industry by growing Agrobacterium on sucrose or molasses, which are plant extracts. The fermentation conditions need the right balance of nutrients, particularly the optimal carbon/nitrogen/phosphate ratio. The bacterium produces curdlan only when the nutrient balance is right, and this fine-tuning requirement drives up the cost of curdlan production.
One way to decrease the cost of curdlan production is to modify the bacterium so that it can ignore the nutrient balance. In this way, curdlan production occurs whenever the bacterium encounters sufficient carbon. The advantage of this approach is that the bacterium can produce high amounts of curdlan from agricultural waste, regardless of the nutrient ratios. The outcome will be (much) cheaper curdlan.
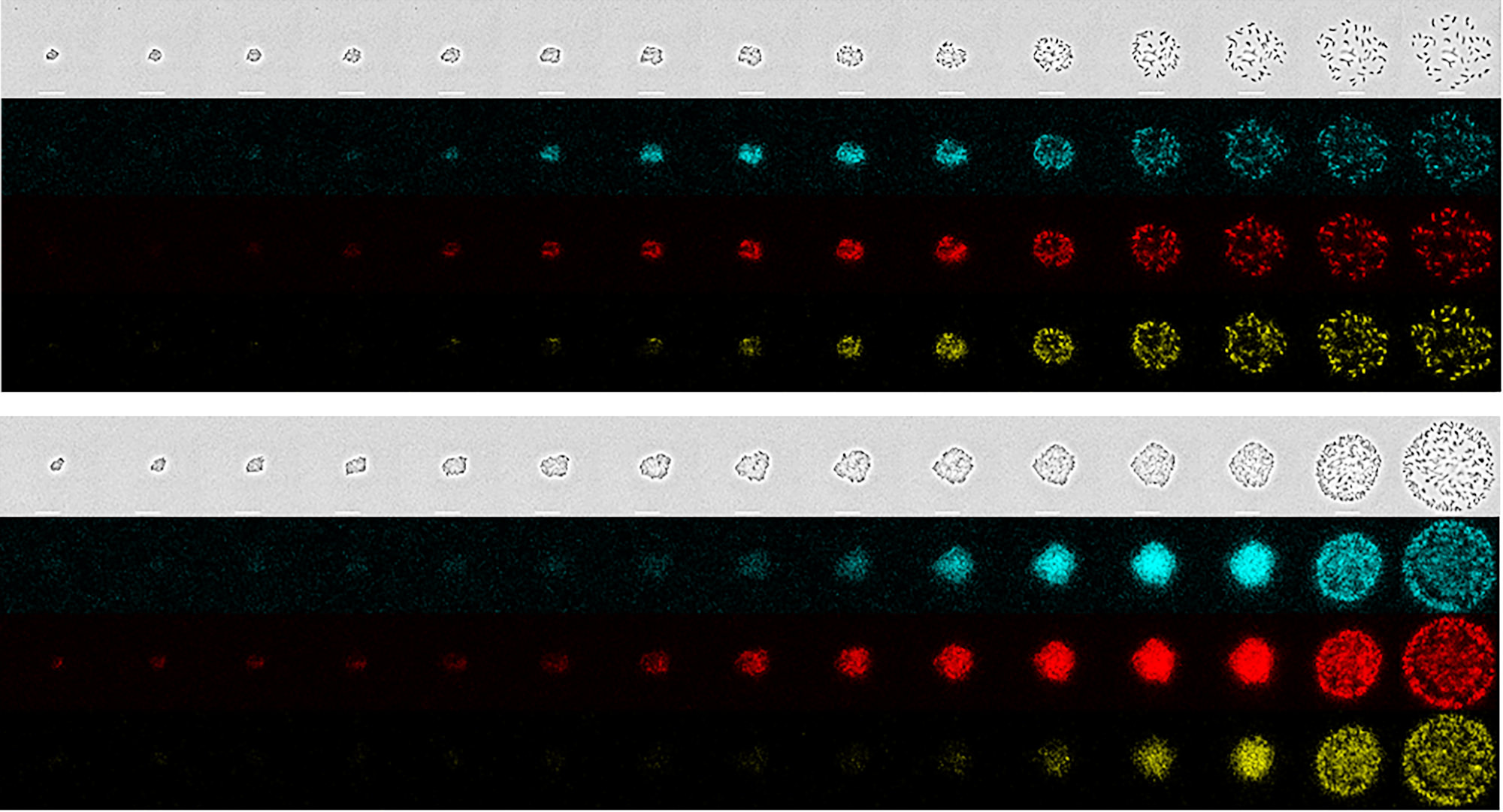
Quorum sensing as a system of production in R. sphaeroides
Quorum sensing is a form of communication between bacteria within a population. Information that is communicated relates to the most important decisions facing the bacteria, e.g., is there enough food available, how large is the population, is the current food availability status suited to a survival strategy of staying home and building a biofilm, or would it be better to produce flagella and move away in search of better environments. In other words, quorum sensing is all about bacterial survival.
From the human view point, quorum sensing regulation of gene expression is very interesting because it represents the most powerful forms of gene expression. Of all the genes expressed by the bacterium, the genes related to quorum sensing have the highest capacity for strong expression. Exactly this capacity is very useful when harnessing bacterial production capacity. This project is focussed on characterizing quorum sensing in Rhodobacter sphaeroides in order to harness its power for maximum production.
A series of photos (one per hour) of bacterial colonies as they grow and start to engage in quorum sensing, followed by moving outwards from the colony – © Matthew McIntosh

Identifying marine bacteria that are efficient at PHB degradation
PHB can replace many forms of plastic that is so prevalent in modern society. If this replacement is successful, we can expect that PHB waste will accumulate in our oceans. How will this affect our marine environments? Will this encourage large populations of bacteria that are specialized at degrading PHB, and if so, does this present a danger for the marine ecosystem.
